Anti-aging through diet
Anti-aging is widely recognized not merely as a means of preventing aging, but as a comprehensive concept encompassing the preservation of overall health. At our laboratory, we aim to practice anti-aging through nutrition by conducting research on the inevitable "decline in health" that comes with aging and the "functional properties of food" that help prevent it. Regarding health deterioration, we are engaged in fundamental research on the formation and role of abnormal modifications—often likened to rust—that have recently attracted attention.
In terms of anti-aging through diet, we focus on the body's innate defense mechanisms: detoxification and innate immunity. We are exploring food components that enhance these protective functions and analyzing their molecular mechanisms. Furthermore, we have launched a new line of research investigating the anti-aging potential of nucleic acids and nucleic acid-related proteins. Through this work, we hope to share the appeal of anti-aging research widely and pioneer a new, original field of study.
About Us
In the spring of 1512, Spanish explorer Juan Ponce de León set sail for the Caribbean in search of a mysterious treasure: the legendary “Fountain of Youth.” Having attained many things in life, he longed for one final prize—eternal life. Driven by this dream, he journeyed from island to island in pursuit of something that likely never existed. Ultimately, he abandoned the quest.
Much like this Spanish explorer, the desire to remain youthful and energetic is a timeless, universal wish. Yet, aging is inevitable. Some may feel it through changes in appearance, while others notice it through fatigue, diminished physical ability, or shifts in sensory functions like sight and hearing. These changes often serve as reminders of aging, and declining health can stir anxiety about illnesses such as cancer.
Although aging cannot be avoided, we can strive to protect our health. Everyone understands that maintaining health requires not only exercise but also proper nutrition. In April of Reiwa 7 (2025), the Advanced Anti-Aging Research Program for Social Collaboration launched its research activities with support from FORDAYS Co., Ltd. Anti-aging research offers many perspectives, but by approaching both “health decline” and “functional properties of food,” we aim to pursue the dream of developing “health-preserving nutrition”—a modern alternative to the mythical “Fountain of Youth” sought by Ponce de León.

(quot. Finkel, T. Nature 425, 132-133 (2003))
Research
Rust Production
60,000 times a day—this is the estimated number of genetic damages sustained by a single cell. Such damage can be caused by a variety of factors and is an unavoidable phenomenon throughout life. One contributing factor is the natural modification of biomolecules.
For example, oxygen and glucose—both essential for survival—are key agents of natural modification. Oxygen exists in a highly reactive biradical state and serves as the origin of various reactive oxygen species. It also plays a critical role in the free radical chain reactions involved in fatty acid peroxidation. Meanwhile, reducing sugars like glucose exhibit reactivity with biomolecules such as proteins due to their aldehyde properties.
Thus, in order to produce energy efficiently, humans rely on oxygen and glucose, and in exchange, must accept the natural modification of biomolecules. This means that the decline of biological functions—aging—is inevitable. Our current research interest lies in the structure and function of naturally modified proteins. Understanding the nature of abnormal modifications associated with aging is essential to advancing anti-aging research.
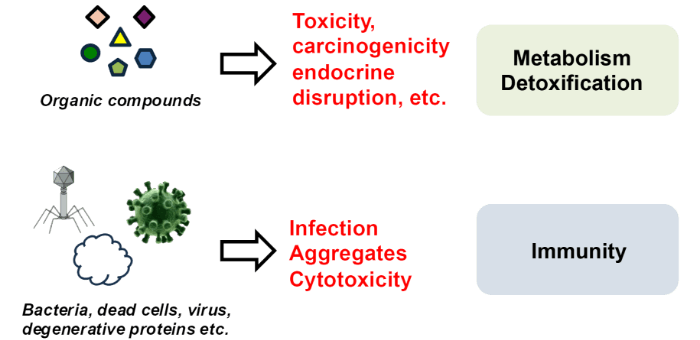
Natural Healing PowerDetoxification and Innate Immunity
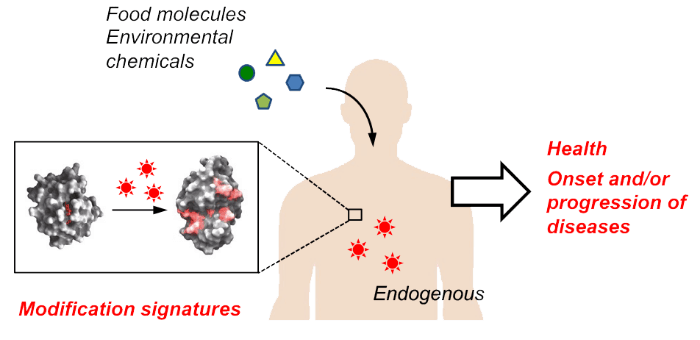
Food is said to have three distinct functions: primary, secondary, and tertiary. The primary function refers to the essential role of food in sustaining life and supporting physical activity. The secondary function involves sensory aspects such as taste, smell, and palatability. The tertiary function relates to maintaining health through physiological regulation and biological defense mechanisms. Anti-aging falls under this third category. Despite its growing social attention due to its connection with health and disease, the specific mechanisms of this function remain poorly understood.
Moreover, food components associated with the tertiary function are often prioritized for application and commercialization, while their fundamental roles and molecular mechanisms are frequently overlooked. Our laboratory is particularly interested in the anti-aging properties of metabolites such as polyphenols and sulfur-containing compounds, which originally serve roles in wound healing and biological defense in plants. We focus on how these food-derived components interact with innate biological defense systems—such as detoxification and immunity—which are naturally present in the human body from birth. This perspective guides our ongoing research.
Functionality of Nucleic Acids and Nucleic Acid-Associated Proteins

Nucleic acids are molecules composed of DNA, which carries genetic information, and the surrounding proteins. But what functions might they serve when consumed as part of the diet? Building on our previous research into the relationship between food and innate immunity, our laboratory has formulated a working hypothesis: that nucleic acids and nucleic acid-associated proteins may possess anti-aging properties. Based on this hypothesis, we have launched a new line of research to explore their potential roles in promoting health and longevity.
Publication
- Original Papers
-
-
Yamaguchi, K., Hu, Y. Y., Kawajiri, K., Itakura, M., Nakashima, F., Shibata, T., Uchida, K. (2025) Adductome-based identification of lysine mono-methylation as a key post-translational protein modification in autoimmune diseases. J. Biol. Chem. 110684.
-
Saimoto, Y., Kusakabe, D., Morimoto, K., Matsuoka, Y., Kozakura, E., Kato, N., Tsunematsu, K., Umen,o T., Kiyotani, T., Matsumoto, S., Tsuji, M., Hirayama, T., Nagasawa, H., Uchida, K., Karasawa, S., Jutanom, M., Yamada, KI (2025) Lysosomal lipid peroxidation contributes to ferroptosis induction via lysosomal membrane permeabilization. Nat. Commun. 16(1), 3554-3554.
-
Itakura, M., Yamaguchi, K., Uchida, K. (2025) Moonlight function of antioxidants. Biosci. Biotech. Biochem. 89(2), 187-192.
-
Morimoto, A., Takasugi, N., Pan, Y., Kubota, S., Dohmae, N., Abiko, Y., Uchida, K., Kumagai, Y., and Uehara, T. (2024) Methyl vinyl ketone and its analogs covalently modify PI3K and alter physiological functions by inhibiting PI3K signaling. J. Biol. Chem. 300,105679.
-
Anan, Y., Itakura, M., Shimoda, T., Yamaguchi, K., Lu, P., Nagata, K., Dong, J., Ueda, H., and Uchida, K. (2024) Molecular and structural basis of anti-DNA antibody specificity for pyrrolated proteins. Commun. Biol. 7, 149.
-
Qiu, B., Zandkarimi, F., Saqi, A., Castagna, C., Uchida, K., Toyokuni, S., Miorin, L., Hibshoosh, H., and Stockwell, B. R. (2024) Fatal COVID-19 pulmonary disease involves ferroptosis. Nat. Commun. 15, 3816.
-
Sei, H., Hirade, N., Kamiya, K., Nakashima, F., Yoshitake, J., Kano, K., Mishiro-Sato, E., Kikuchi, R., Uchida, K., Shibata, T. (2024) Isocitrate dehydrogenase 1 upregulation in urinary extracellular vesicles from proximal tubules of type 2 diabetic rats. FASEB J.38(10): e23688.
-
Sasayama, Y., Mamiya, T., Qi, J., Shibata, T., Uchida, K., Nabeshima, T., and Ojika, M. (2023) Neuritogenic steroid glycosides from crown-of-thorns starfish: possible involvement of p38 mitogen-activated protein kinase and attenuation of cognitive impairment in senescence-accelerated mice (SAMP8) by peripheral administration. Bioorg. Med. Chem. 78, 117144.
-
Hirayama, A., Hayasaka, R., Tabata, S., Hasebe, M., Ikeda, S., Hikita, T., Oneyama, C., Yoshitake, J., Onoshima, D., Takahashi, K., Shibata, T., Uchida, K., Baba, Y., Soga, T., and Tomita, T. (2023) Metabolomics of small extracellular vesicles derived from isocitrate dehydrogenase 1-mutant HCT116 cells collected by semi-automated size exclusion chromatography. Front. Mol. Biosci., section Molecular Diagnostics and Therapeutics. 9:1049402.
-
Nakahara, K., Okuda, K., Ito, A., Kumar, A., Nomura, R., Iijima, Y., Takasugi, KN., Adachi, K., Shimada, Y., Fujio, S., Onuma, K., Osaki, M., Okada, F., Ukegawa, T., Takeuchi, Y., Yasui, N., Yamashita, A., Marusawa, H., Katagiri, T., Shibata, T., Uchida, K., Nakamura, T., Zhang, K. Y. J., Lipton, S. A., and Uehara, T. (2023) Pivotal role for S-nitrosylation of DNA methyltransferase in epigenetic regulation. Nat. Commun. 14, 621.
-
Yoshitake, J., Shibata, T., Chikazawa, M., and Uchida, K. (2023) Autoxidation of ascorbate mediates lysine N-pyrrolation. Free Radic. Res. 56, 749-759.
-
Nakashima, F., Loh, W. Q., Wakabayashi, M., Shimomura, H., Hattori, H., Kita, M., Inoue, A., Uchida, K., Shibata, T. (2023) Eriodictyol and thymonin act as GPR35 agonists. Biosci. Biotech. Biochem. 87, 1514-1522.
-
Nishiyama, K., Nishimura, A., Shimoda, K., Tanaka, T., Kato, Y., Shibata, T., Tanaka, H., Kurose, H., Azuma, Y. T., Ihara, H., Kumagai, Y., Akaike, T., Eaton, P., Uchida, K., and Nishida, M. (2022) Redox-dependent internalization of purinergic P2Y6 receptor limits colitis progression. Sci. Signal. 15, eabj0644.
-
Lim, S.Y., Yamaguchi, K., Itakura, M., Chikazawa, M., Matsuda, T., and Uchida, K. (2022) Unique B-1 cells specific for both N-pyrrolated proteins and DNA evolve with apolipoprotein E deficiency. J. Biol. Chem. 298, 101582.
-
Itakura, M., Yamaguchi, K., Kitazawa, R., Lim, S. Y., Anan, Y., Yoshitake, J., Shibata, T., Negishi, L., Sugawa, H., Nagai, R., and Uchida, K. (2022) Histone functions as a cell-surface receptor for AGEs. Nat. Commun. 13, 2974.
-
Yoshitake, J., Azami, M., Sei, H., Onoshima, D., Takahashi, K., Hirayama, A., Uchida, K., Baba, Y., and Shibata, T. (2022) Rapid isolation of extracellular vesicles using a hydrophilic porous silica gel-based size-exclusion chromatography column. Anal. Chem. 94, 13676-13681.
-
Yamaguchi, K., Itakura, M., Tsukamoto, M., Lim, S.Y., and Uchida, K. (2022) Natural polyphenols convert proteins into histone-binding ligands. J. Biol. Chem. 298, 102529.
-
Uchida, K. (2022) Conversion of proteins into DNA mimetics by lipid peroxidation. Arch. Biochem. Biophys. 728, 109374.
-
Komae, S., Kasamatsu, S., Uchida, K., and Ihara, H. (2022) Quantitative determination of 2-oxo-Imidazole-containing dipeptides by high-performance liquid chromatography/tandem mass spectrometry. Antioxidants 11, 2401.
-
Hasegawa, K, Kuwata, K., Yoshitake, J., Shimomura, S., Uchida, K., Shibata, T. (2021) Extracellular vesicles derived from inflamed murine colorectal tissue induce fibroblast proliferation via epidermal growth factor receptor. FEBS J. 288, 1906–1917.
-
Endo, R., Uchiyama, K., Lim, S. Y., Itakura, M., Adachi, T., and Uchida, K. (2021) Recognition of acrolein-specific epitopes by B cell receptors triggers an innate immune response. J. Biol. Chem. 296, 100648-100648.
-
Tisma, V. S., Bulimbasic, S., Ljubanovic, D. G., Galesic, K., Vergles, J. M., Mitrovic, J., Uchida, K., Tatzber, F., Zarkovic, N., Jaganjac, M. (2021) The onset of systemic oxidative stress associated with accumulation of lipid peroxidation product acrolein in the skin of patients with small-vessel vasculitis. Mol. Med. 26(8):2344.
-
Yamaguchi, K., Itakura, M., Kitazawa, R., Lim, S. Y., Nagata, K., Shibata, T., Akagawa, M., and Uchida, K. (2021) Oxidative deamination of lysine residues by polyphenols generates an equilibrium of aldehyde and 2-piperidinol products. J. Biol. Chem. 297, 101035.
-
Kakihana, Y., Kasamatsu, S., Yamakage, A., Uchida, K., and Ihara, H. (2021) Distribution and quantitative analysis of homoanserine and its 2-oxo derivative in mouse tissues. Free Radic. Res. 55, 688-697.
-
Tanaka, H., Hosoi, Y., Ishikawa, K., Yoshitake, J., Shibata, T., Uchida, K., Hashizume, H., Mizuno, M., Okazaki, Y., Toyokuni, S., Nakamura, K., Kajiyama, H., Kikkawa, F., and Hori, M. (2021) Low temperature plasma irradiation products of sodium lactate solution that induce cell death on U251SP glioblastoma cells were identified. Sci. Rep. 16, 18488.
-
Katayoshi, T., Kusano, Y., Shibata, T., Uchida, K., and Tsuji-Naito, K. (2021) Low-molecular-weight whey proteins promote collagen production in dermal fibroblasts via the TGF-β receptor/Smad pathway. Biosci. Biotech. Biochem. 85, 2232-2240.
-
Kasamatsu, S., Komae, S., Matsukura, K., Kakihana, Y., Uchida, K., and Ihara, H. (2021) 2-Oxo-imidazole-containing dipeptides play a key role in the antioxidant capacity of imidazole-containing dipeptides. Antioxidants 10, 1434.
-
Erdélyi, K., Ditrói, T., Johansson, H. J., Czikora, A., Balog, N., Silwal-Pandit, L., Ida, T., Olasz, J., Hajdú, D., Mátrai, Z., Csuka, O., Uchida, K., Tóvári, J., Engebraten, O., Akaike, T., Børresen Dale, A. L., Kásler, M., Lehtiö , J., and Nagy, P. (2021) Reprogrammed transsulfuration promotes basal like breast tumor progression via realigning cellular cysteine-persulfidation. Proc. Natl. Acad. Sci. U.S.A. 118, e2100050118.
-
Fujikawa, K., Nakahara, K., Takasugi, N., Nishiya, T., Ito A., Uchida, K., and Uehara, T. (2020) S-Nitrosylation at the active site decreases the ubiquitin-conjugating activity of ubiquitin-conjugating enzyme E2 D1 (UBE2D1), an ERAD-associated protein. Biochem. Biophys. Res. Commun. 524, 910-915.
-
Petkovic, I., Bresgen, N., Gilardoni, E., Regazzoni, L., Uchida, K., Aldini, G., Siems, W., Eckl, P. (2020) In vitro aging of human skin fibroblasts: Age-dependent changes in 4-hydroxynonenal metabolism. Antioxdiants 9, 150.
-
Feng, H., Schorpp, K., Jin, J., Yozwiak, C. E., Hoffstrom, B. G., Decker, A. M., Rajbhandari, P., Stokes, M. E., Bender, H. G., Csuka, J. M., Upadhyayula, P. S., Canoll, P., Uchida, K., Hadian, K., and Stockwell, B. R. (2020) Transferrin receptor is a specific ferroptosis marker. Cell Rep. 30, 3411-3423.
-
Sasatsuki, H., Nakazaki, A., Uchida, K., and Shibata, T. (2020) Quantitative analysis of oxidized vitamin B1 metabolites generated by hypochlorous acid. Free Radic. Biol. Med. 152, 197-206.
-
Chikazawa, M., Yoshitake, J., Lim, S.-Y., Iwata, S., Negishi, L., Shibata, T., and Uchida, K. (2020) Glycolaldehyde is an endogenous source of lysine N-pyrrolation. J. Biol. Chem. 295, 7697-7709.
-
Luong, N. C., Abiko, Y., Shibata, T., Uchida, K., Warabi, E., Suzuki, M., Noguchi, T., Matsuzawa, A., and Kumagai, Y. (2020) Redox cycling of 9,10-phenanthrenequinone activates epidermal growth factor receptor signaling through S-oxidation of protein tyrosine phosphatase 1B. J. Toxicol. Sci. I45, 349-363.
-
Takamiya, R., Takahashi, M., Maeno, T., Saito, A., Kato, M., Shibata, T., Uchida, K., Ariki, S., Nakano, M. (2020) Acrolein in cigarette smoke attenuates the innate immune responses mediated by surfactant protein D. Biochim. Biophys. Acta 1864, 129699.
-
Ishii, I., Kamata, S., Oyama, T., Saito, K., Honda, A., Yamamoto, Y., Suda, K., Ishikawa, R., Itoh, T., Watanabe, Y., Shibata, T., Uchida, K., and Suematsu, M. (2020) PPARα ligand-binding domain structures with endogenous fatty acids and fibrates. iScience 23, 101727.
CLOSE
-
- Reviews & Books
-
-
中島史恵、柴田貴広、内田浩二 (2024) “食品成分によるタンパク質修飾を介した炎症抑制活性” 日本栄養・食糧学会誌. 77, 125-130.
-
板倉正典、内田浩二 (2022) “タンパク質最終糖化産物 (AGEs)の抗炎症機能” カレントトピックス 実験医学 羊土社 Vol.40, 2637-2640.
-
Niki, E and Uchida, K. (2021) Special issue on “Recent Topics of Redox Chemistry and Biology”. Free Radic. Res. 55, 305-306.
-
Naito, Y., Toyokuni, S., and Uchida, K. (2020) The new era for redox research. Free Radic. Res. 26, 1-7.
内田浩二 (2020) “序に変えて:「医食同源」を解明する食と健康研究の最前線” 実験医学増刊「食と健康を結ぶメディカルサイエンス」 (内田浩二 編集) 羊土社 Vol. 38.
内田浩二 (2020) “リポキシデーションによるタンパク質の自然修飾” 実験医学増刊「食と健康を結ぶメディカルサイエンス」 (内田浩二 編集) 羊土社 Vol. 38, 1605-1609.
中島史恵、柴田貴広、内田浩二 (2020) “システイン残基におけるユニークな自然修飾” 実験医学増刊「食と健康を結ぶメディカルサイエンス」 (内田浩二 編集) 羊土社 Vol. 38, 1622-1628.
CLOSE
-
- Conference Presentations
-
内田 浩二
疾患に関連した翻訳後修飾シグネチャーの構造と機能
第50回日本医用マススペクトル学会年会
2025年9月(一宮)Koji Uchida
Chemoprevention by foods
Redox Chemistry and Biology for Drug Development and Health Management
2025年2月(木甫、韓国)Koji Uchida
Immune memory against toxic aldehydes
The 11th Biennial Meeting of the Society for Free Radical Research-Asia (SFRR-Asia) and the Chinese National Conference of Redox Biology and Medicine 2024
2024年10月(北京、中国)Koji Uchida
Immune memory against toxic aldehydes
HNE club
2024年10月(ジェノバ、イタリア)内田 浩二
毒性アルデヒドに対する免疫記憶(特別講演)
第51回日本毒性学会学術年会
2024年7月(博多)内田 浩二
シンポジウム レドックスシグネチャーの生物学 イントロダクション
第96回日本生化学会大会
2023年11月(博多)内田 浩二
マクロファージによるapoE受容体を介したピロール化タンパク質の取り込み
第96回日本生化学会大会
2023年10月(博多)内田 浩二
シンポジウム 付加体科学とは何か 短寿命活性種に起因する付加体の構造と機能
第96回日本生化学会大会
2023年10月(博多)内田 浩二
付加体発掘40年(特別講演)
日本毒性学会付加体科学部会第1回キックオフシンポジウム
2023年9月(岡山)内田 浩二
シンポジウム 化学物質のアダクト形成を介した新規毒性機構の解明とその検出 酸化に起因した修飾シグネチャーの構造と機能
第50回日本毒性学会学術年会
2023年6月(横浜)内田 浩二
シンポジウム1農学系研究者と医学系研究者からの発信~連携に向けて~、抗酸化剤に起因する修飾シグネチャーの構造と機能
第76回日本酸化ストレス学会学術集会
2023年5月(神戸)内田浩二
2-オキソヒスチジンの発見
第76回日本栄養・食糧学会大会シンポジウム「イミダゾールペプチド研究の新展開」2022年6月(神戸)内田浩二
自然免疫に関与する酸化特異的エピトープ
第64回日本脂質生化学会
2022年6月(東京)内田浩二
タンパク質修飾を介した食の免疫記憶
第49回日本毒性学会学術年会 ワークショップ「エクスポソームの新戦略」
2022年7月(札幌)内田 浩二
酸化ストレス疾患病態解析における分析技術の新展開
第74回日本酸化ストレス学会・第21回日本NO学会合同学術集会
2021年5月(オンライン)内田浩二
抗化剤機能のパラダイムシフト
日本農芸化学会2021年度大会「抗酸化研究の新展開」シンポジウム
2021年3月(オンライン)内田浩二
エキスポソームに備える生体防御系としての自然抗体
第94回日本薬理学会年会「薬理学・毒性学視点からアプローチするエクスポソーム研究」シンポジウム
2021年3月(札幌)内田浩二
リポキシデーションによるタンパク質自然修飾
第73回日本酸化ストレス学会「新しい酸化脂質解析法を用いたオキシリピッドバイオロジー研究」シンポジウム
2020年10月(オンライン)内田浩二
ユニークなタンパク質修飾反応リポキシデーション
第93回 日本生化学会大会「新たな酸化脂質研究の潮流」シンポジウム
2020年9月(オンライン)
CLOSE
Member
Contact
Advanced Anti-aging Research Center,
Graduate School of Agricultural and Life Sciences,
The University of Tokyo,
1-1-1, Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan.
(Please change "[at]" to "@" before sending.)